Takeaways
- Dual-Path Weight Loss cuts fat dramatically while preserving lean muscle, solving the key drawback of GLP-1 monotherapy.
- Phase 2b BELIEVE data show 22 % total-weight loss with 93 % fat reduction; a Phase 3 program begins soon.
- This combo could reshape obesity care, so clinics should refine monitoring, nutrition, and infusion workflows now.
Setting the Stage: The Muscle-Loss Problem in Modern Obesity Care
Clinicians embrace modern GLP-1 drugs for weight control within physician-guided medical weight loss programs yet remain alarmed by collateral muscle loss. DEXA studies show up to a third of lean tissue vanishes during monotherapy. The 2025 BELIEVE Phase 2b late-breaker abstract reported twenty-two percent weight loss with ninety-three percent from fat. These data spotlight bimagrumab plus semaglutide as the first regimen that protects muscle while shredding fat. Our discussion explores the science, safety signals, and practical clinic workflows behind this dual-path innovation.
Meet the Dual-Path Concept
Researchers coined the term dual-path because the combination addresses both energy intake and tissue remodeling. Semaglutide reduces calorie consumption by boosting satiety and slowing gastric emptying. Bimagrumab simultaneously triggers myofiber growth, raising resting metabolic rate despite caloric deficit. Consequently, fat depots shrink while muscle stays functional, a result unmatched by single-agent therapies. The following subsections unpack each pathway and illustrate their remarkable synergy.
Mechanism 1: GLP-1 Appetite & Glycemic Control
Semaglutide acts as a long-acting GLP-1 analog that intensifies insulin release after meals. Enhanced insulin sensitivity flattens post-meal glucose spikes, cutting hunger rebound later in the day. Slower gastric emptying prolongs satiety, so patients voluntarily trim portions without rigid dieting. Reduced glucose variability dampens inflammatory cytokines that otherwise promote visceral adiposity. These effects collectively create a sustainable energy deficit yet place muscle mass at risk.
Mechanism 2: ActRII Blockade for Anabolic Rescue
Bimagrumab blocks activin type II receptors expressed on skeletal muscle cells. This blockade lifts myostatin’s brake on protein synthesis, allowing mTOR signaling to surge. Resulting hypertrophy offsets catabolism that normally accompanies aggressive dieting. Animal studies demonstrate higher mitochondrial density and enhanced fatty-acid oxidation after treatment. Early human data mirror these findings, showing lean-mass gain despite reduced caloric intake. Such anabolic support forms the second lane of the dual-path strategy.
Synergy Diagram & Analogy (call-out graphic)
Picture a hybrid vehicle that sips fuel yet gains horsepower from electric boost. Semaglutide supplies the efficiency component by minimizing intake. Bimagrumab delivers the horsepower, expanding muscle and incinerating fat at rest. Two parallel arrows on a clinic handout can convey this simple principle to patients. The illustration clarifies why the duo outperforms any existing monotherapy.
Inside the BELIEVE Trial
Investigators launched BELIEVE to translate laboratory synergy into real-world outcomes across diverse populations. Enrollment spanned ten countries, capturing varied metabolic phenotypes and ethnic backgrounds. Participants underwent baseline DEXA scans, biomarker panels, and functional assessments. The randomized nine-arm design on ClinicalTrials.gov enables robust head-to-head comparisons. Weekly visits monitored adverse events and medication adherence with digital injection trackers.
Design & Dosing Schedule
Semaglutide titrated to 2.4 mg weekly, matching commercial obesity protocols for injectables. Bimagrumab infusions occurred every four weeks at body-weight-adjusted doses. Participants and staff remained blinded through matched saline infusions and dummy pens. Investigators offered minimal lifestyle guidance to isolate pharmacologic effects accurately. Adherence exceeded ninety percent, aided by user-friendly reminder apps.
Primary & Secondary Endpoints
The primary endpoint measured percent change in total fat mass at week forty-eight. Lean-mass preservation, waist circumference, and liver fat comprised hierarchical secondary outcomes. Researchers also tracked inflammatory C-reactive protein and quality-of-life scores. Exploratory endpoints included stair-climb time and hand-grip strength. This comprehensive battery paints a holistic picture of body composition and function.
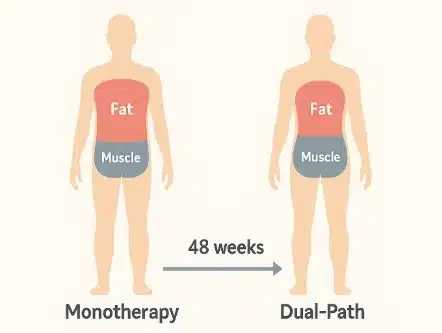
Before-and-after silhouette shows how dual-path therapy trims fat while safeguarding muscle over 48 weeks.
Top-Line Efficacy Results
High-dose combination therapy reduced body weight by 22.1 percent, eclipsing semaglutide alone. DEXA scans showed ninety-three percent of that loss came from fat, confirming muscle protection. Nearly seventy percent of participants achieved at least twenty percent weight loss. Waist circumference and liver fat declined more sharply versus all comparator arms. Detailed results appear in the ADA abstract, reinforcing statistical significance across endpoints.
Safety Profile and Lab-Signal Deep Dive
Adverse events mirrored standard GLP-1 therapy, with transient nausea and mild diarrhea leading counts. Investigators noted temporary LDL-cholesterol rises, yet semaglutide co-administration blunted that pattern. Liver enzymes stayed within normal ranges, and creatine kinase remained stable, supporting genuine anabolism. A JAMA cohort study showed GLP-1 monotherapy sacrifices one-third of lean mass. At Fountain of Youth SWFL, clinicians pair DEXA scanning with lipid panels to protect patient safety.
How Does It Stack Up Against Other Combos?
Tirzepatide delivers impressive scale numbers, yet it erodes muscle more severely than the dual-path approach. Cagrilintide combined with semaglutide enhances satiety, although body-composition scans still reveal muscle depletion. Early trevogrumab data preserve muscle better, yet fat loss remains modest relative to bimagrumab. Scholar Rock’s myostatin inhibitor aims to rebuild muscle after GLP-1 discontinuation rather than during therapy. Our team at Fountain of Youth SWFL translates these comparative findings into clear visuals during consultations.
Clinical Translation in 2025–2027
Phase 3 enrollment begins soon, so clinics should evaluate workflows now. Many offices already administer weekly injections, yet monthly infusions demand dedicated space and scheduling. The upcoming subsections outline candidate selection, payer obstacles, and lifestyle synergy. Early adopters may join registry studies to access medication before commercial launch. Patient education must emphasize both benefits and remaining unknowns to sustain trust.
Ideal Candidate Profiles
Sarcopenic obesity patients over fifty gain most because preserved muscle directly reduces fall risk. Competitive athletes cutting weight value strength retention during demanding training cycles. Older women with osteopenia benefit from anabolic effects that also support bone density. Patients reporting low stamina sometimes request an energy restoration infusion for quick vitality, although more data will guide future protocols. Clinicians still exclude individuals with severe renal impairment until further safety data emerge.
Insurance & Access Hurdles
Payers hesitate at current GLP-1 costs, so adding a biologic magnifies reimbursement scrutiny. Value-based contracts may tie coverage to documented lean-mass preservation rather than simple weight loss. Employer plans could reward clinics that show improved functional metrics such as chair-rise time. Trial participation offers drug supply without cost, easing access for motivated candidates. Medicare policy will likely hinge on fracture-outcome data expected post-approval.
Nutrition & Resistance-Exercise Adjuncts
Protein intake should reach at least 1.2 grams per kilogram to support synthesis. Leucine-rich foods and periodic creatine loading further optimize anabolic signaling. A session with performance IV drips can reduce soreness while replacing amino acids lost during hard workouts. Some athletes also rely on a classic Myers Cocktail for micronutrient balance between sessions. Clinicians monitor hydration, and regular vitamin injections maintain micronutrient stores when rapid fat loss complicates diet quality.
Adding a clear monitoring grid helps clinicians track safety and efficacy once dual-path therapy enters routine care. Use the schedule below as a quick-glance checklist during follow-up visits.
| Parameter | Baseline Timing | Follow-Up Interval | Action Threshold |
|---|---|---|---|
| DEXA Scan | Within 2 weeks pre-start | Every 6 months | >2 % lean-mass loss triggers physical-therapy referral |
| Lipid Panel | Day 0 (fasting) | Every 3 months | LDL rise >30 mg/dL warrants statin discussion |
| Comprehensive Metabolic Panel | Day 0 | Every 6 months | ALT or AST >2× ULN prompts hepatology consult |
| Creatine Kinase | Day 0 | At 12 weeks, then annually | CK >1,000 U/L signals dose review |
| Functional Strength Test (Chair-Rise Time) |
Within first visit | Every 6 months | Time increase >20 % demands resistance-training tweak |
Regulatory & Commercial Outlook
Developers intend to request fast-track designation under the FDA obesity-drug guidance. Regulators may accept lean-mass change as a surrogate while mandating fracture surveillance post-launch. Analysts predict pricing on par with current GLP-1s plus moderate infusion fees. Specialty pharmacies will manage cold-chain logistics, ensuring antibody stability during shipping. Future payer bundles might combine dual-path therapy with supportive wellness services for older adults.
3 Practical Tips for Practitioners
- Secure a baseline DEXA scan before prescribing to document lean-mass status objectively.
- Establish protein goals early and revisit them during every follow-up visit.
- Schedule lipid testing at three-month intervals to track transient cholesterol shifts.
- Encourage resistance training because mechanical loading enhances pharmacologic muscle gains.
- Use shared decision aids that outline benefits, risks, and monitoring plans in plain language.
FAQ
Will muscle-preserving combos cost more than GLP-1s alone?
Analysts expect higher upfront costs because infusion fees and biologic manufacturing increase total expenditure. Nevertheless, avoided sarcopenia may reduce rehabilitation expenses, potentially balancing payers’ long-term budgets.
Could ActRII blockade worsen heart failure?
Early trials excluded advanced heart-failure patients, and no signal emerged among enrolled participants. Phase 3 will include echocardiography, so future data will clarify cardiac safety comprehensively.
Is IV delivery a deal-breaker for primary care?
Monthly infusions pose logistical hurdles, yet many primary offices already manage biologic therapies for rheumatology. Shared infusion suites or coordinated specialty centers can fill gaps until in-office capability scales up.
What happens to muscle mass after therapy stops?
Researchers continue monitoring participants off-drug, yet preliminary data suggest muscle remains stable for months. Longer observations will reveal whether resistance training and adequate protein maintain gains indefinitely.
Key Takeaways
Dual-path therapy marries appetite suppression with direct anabolic signaling for unmatched body-composition outcomes. Phase 2b data confirm dramatic fat loss alongside near-complete muscle preservation. Safety signals look manageable, although lipid trends warrant ongoing monitoring. Competing combos still sacrifice lean tissue, positioning bimagrumab plus semaglutide at the forefront. Clinics that prepare protocols now will deliver superior results when approvals arrive.
References
- American Diabetes Association. “2180-LB: Bimagrumab Augments Metabolic Rate to Improve Incretin-Based Weight Loss.” diabetesjournals.org.
- National Library of Medicine. “Safety and Efficacy of Bimagrumab + Semaglutide in Adults With Obesity.” clinicaltrials.gov.
- NIH-PubMed. “Antibody Blockade of Activin Type II Receptors Preserves Skeletal Muscle Mass.” pubmed.ncbi.nlm.nih.gov.
- JAMA Internal Medicine. “Semaglutide vs Tirzepatide for Weight Loss in Adults With Overweight/Obesity.” jamanetwork.com.
- U.S. Food and Drug Administration. “Obesity and Overweight: Developing Drugs and Biological Products for Weight Reduction.” fda.gov.
Medical review: Reviewed by Dr. Keith Lafferty MD, Fort Myers on July 5, 2025. Fact-checked against government and academic sources; see in-text citations. This page follows our Medical Review & Sourcing Policy and undergoes updates at least every six months.