Here’s what you’ll learn when you read this article:
-
Why adding long-acting PYY to GLP-1 therapy showed only modest benefits and meaningful tolerability limits in human studies.
-
How to interpret add-on obesity drug trials without being misled by headlines or study design details.
-
What the PYY1875 evidence means for real patients considering combination approaches today.
What Patients Should Know About PYY1875
Weight-loss medications built around GLP-1 receptors have changed what many people expect from obesity treatment. For some patients, appetite quiets, portions shrink naturally, and meaningful weight loss becomes possible after years of frustration. For others, progress slows, side effects limit dose increases, or hunger returns sooner than expected. That reality has pushed researchers to look beyond GLP-1 alone and ask whether combining different satiety signals could help.
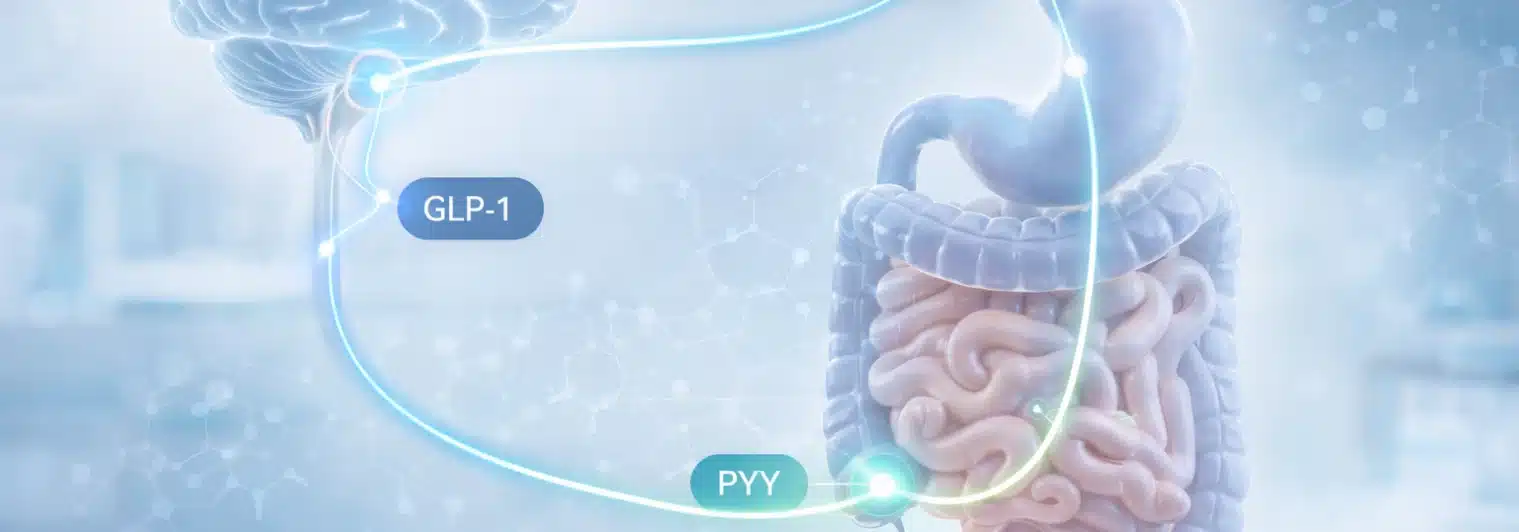
One approach now re-emerging involves Peptide YY (PYY), a naturally occurring gut hormone that also influences appetite. In recent years, scientists have developed long-acting PYY 3–36 analogs, including one called PYY1875, designed to last long enough in the body to be practical as medication. Clinical trials have tested PYY1875 both on its own and as an add-on to semaglutide, a widely used GLP-1 drug.
This article explains what PYY is, why long-acting versions matter, what the PYY1875 studies actually showed, and how patients can interpret this information realistically. The goal is clarity, not hype.
Why PYY Is Back in the Conversation
Peptide YY is not new. The hormone has been studied for decades as part of the body’s natural appetite regulation system. PYY’s relevance in obesity pharmacotherapy has resurfaced because researchers can now test a long-acting PYY 3–36 analogue in randomized clinical studies rather than relying on short-acting approaches.
What has changed is not the concept of PYY as a satiety signal, but the ability to turn that concept into a medicine that can be dosed in a way people can realistically use. Earlier attempts struggled with practicality and tolerability, while newer designs aim for sustained action.
What “Long-Acting” Really Means for PYY
Native peptide hormones often break down quickly in the bloodstream, and that limitation can make them impractical for chronic treatment. Long-acting analogs attempt to extend duration and maintain receptor activity long enough for clinically workable dosing schedules.
PYY1875 is a long-acting analogue of PYY3–36 that was evaluated in preclinical work and then moved through early human studies. The development path includes variant screening of PYY 3–36 to support potency, stability, and Y2 receptor selectivity, followed by clinical testing of PYY1875 alone and as an add-on to semaglutide.
Why Combine PYY With GLP-1 Instead of Replacing It?
GLP-1 receptor agonists already influence appetite, digestion, and satiety, and many people benefit from them. Researchers have still explored add-on strategies because obesity biology rarely runs through a single pathway, and complementary signals can sometimes produce incremental benefit.
The PYY1875 program explicitly tested whether a long-acting PYY3–36 analogue could serve as a complementary pharmacotherapy alongside a GLP-1 receptor agonist. The human evidence base is described in the Obesity (2025) report on PYY1875 alone and as an add-on to semaglutide, which covers preclinical assessment through randomized clinical studies.
What the Human Studies on PYY1875 Actually Tested
PYY1875 has been evaluated in early-phase human studies, including randomized clinical studies where it was added to semaglutide in adults with obesity. These trials were not designed to establish long-term efficacy or regulatory readiness, but instead focused on safety, tolerability, dose escalation limits, and whether incremental weight loss beyond semaglutide alone could be demonstrated without unacceptable gastrointestinal side effects.
In the phase 2 add-on design described in the published report, participants received semaglutide first, then were randomized to add-on PYY1875 or placebo while continuing semaglutide. This structure helps separate “ongoing GLP-1 effects” from what the add-on contributes.
How to read PYY add-on trial results without getting misled: These checkpoints help you evaluate whether an “add-on” obesity study shows a real, patient-relevant advantage or mostly reflects study design and tolerability limits. Use the table to compare future PYY or combination-therapy headlines with what actually matters in day-to-day outcomes.
| What to look at | Why it matters for patients | What to ask or check | Red flags in headlines |
|---|---|---|---|
| Run-in period on GLP-1 before add-on | Early weight loss from the GLP-1 can make later “extra loss” look smaller, or can mask who actually benefits from the add-on. | Did everyone reach a stable GLP-1 dose before randomization? How much weight loss happened before the add-on started? | “Add-on caused huge loss” without stating that most loss occurred during GLP-1 run-in. |
| Comparator group (placebo add-on) | Patients need to know what changes beyond continuing the GLP-1 alone. | Was it “add-on vs add-on placebo” while continuing the same GLP-1 regimen? | Comparisons that quietly switch doses or switch background therapy. |
| Outcome definition | “More weight loss” can mean small averages that may not feel meaningful to the individual. | Is the primary endpoint percent weight change, responder rates, or changes in appetite measures? | “Clinically meaningful” claims with no clear threshold or patient-facing interpretation. |
| Dose escalation and tolerability | An add-on only works in real life if people can stay on a dose that produces benefit without unacceptable side effects. | How many people discontinued? Were higher doses limited by nausea or vomiting? | Efficacy highlighted while dose-limiting side effects are minimized or omitted. |
| Timeframe of treatment | Short trials can show early appetite effects but may not predict long-term weight stability or adherence. | How many weeks were studied after the add-on started? Was there follow-up after stopping? | Big claims based on short durations without durability data. |
| Who was included (population) | Results can differ if participants already tolerate GLP-1s well, or if they represent a narrower “trial-ready” group. | Were participants GLP-1 experienced? Were people with significant GI issues excluded? | Assuming results apply to everyone using GLP-1 therapy in real life. |
| What “added benefit” looks like beyond weight | Patients often care about hunger control, cravings, meal satisfaction, and day-to-day function, not only the scale. | Did the study measure appetite, quality of life, or eating behavior outcomes alongside weight? | Only weight loss is reported, with no patient-experience outcomes. |
| Real-world feasibility | Weekly dosing, side effect management, and persistence determine whether an add-on can be realistic outside a trial. | Would a typical patient be able to follow the same titration schedule and monitoring approach? | “Game changer” language without discussing adherence and tolerability in practice. |
What the Results Showed About Weight Loss
The headline finding from the phase 2 data is straightforward: PYY1875 added to semaglutide produced only modest additional weight loss compared with semaglutide alone. Unlike many secondary summaries, the peer-reviewed report explicitly characterizes this effect as modest and notes that gastrointestinal tolerability limited dose escalation, constraining how much additional benefit could realistically be tested.
After participants had already lost weight during semaglutide treatment, adding PYY1875 led to further weight reduction, but the difference between the add-on group and the placebo add-on group was relatively small in the reported randomized clinical studies. The published authors concluded that the add-on effect was modest, and tolerability constraints influenced the regimen that could be tested.
For patients, that distinction matters. Incremental improvement may still be valuable in some contexts, but only if it comes without unacceptable downsides.
Tolerability Turned Out to Be the Limiting Factor
Side effects, particularly gastrointestinal symptoms, emerged as the key challenge. The published clinical report notes that gastrointestinal-related adverse events were common with the 1.0 mg PYY1875 dose and that the 2.0 mg PYY1875 dose escalation regimen was not tolerated when used as an add-on to semaglutide.
That finding matters because combination therapies only make sense if each component can be dosed effectively. When tolerability limits dose escalation, the theoretical benefit of stacking satiety signals becomes harder to realize.
Clinical trial registration information for the combination program is available through NCT04969939, which describes semaglutide in combination with NNC0165-1875 (PYY1875) in people with obesity.
What These Results Mean for Patients Today
From a patient perspective, the most important takeaway is balance. The PYY1875 data show that adding another satiety-related signal is not automatically better, even when the biology makes sense on paper.
Semaglutide already exerts strong effects that many people experience as appetite reduction and changes in food tolerance. The clinical program for PYY1875 suggests that layering another peptide signal on top of semaglutide can run into tolerability limits before delivering a large additional weight-loss effect.
At the same time, these results do not invalidate the concept of combination therapy. They show that this specific approach, as tested, faced a tight tradeoff between modest added benefit and gastrointestinal side effects.
Why the Research Still Matters Despite Modest Results
Modest trials still move the field forward by defining boundaries as clearly as successes. The PYY1875 program demonstrated that a long-acting PYY3–36 analogue can be evaluated in humans through randomized studies, while also clarifying where tolerability and dose escalation constraints limit the practical value of this add-on strategy.
The preclinical-to-clinical development work is detailed in Science Translational Medicine, which describes the identification and characterization of long-acting PYY3–36 analogs, including design features relevant to potency, selectivity, and stability.
These insights can guide future development efforts toward designs and dosing strategies that better balance patient-relevant benefit and tolerability.
How to Interpret Headlines About “Next-Generation” Weight-Loss Drugs
Patients often encounter headlines suggesting that the next combination therapy will dramatically outperform existing drugs. The PYY1875 experience supports a more careful approach: look for patient-relevant incremental benefit and check how side effects shaped dosing.
When evaluating claims about add-on therapies, useful questions include whether the added weight-loss effect is clearly described, whether the comparator group is appropriate, and whether tolerability issues limited what could be tested. The table above provides a quick way to pressure-test future claims against the basics that matter.
Practical Implications for People Using GLP-1 Therapy Now
Patients currently taking GLP-1 medications sometimes wonder whether an add-on could restart weight loss or smooth hunger fluctuations. The PYY1875 clinical findings suggest that add-ons are not guaranteed solutions and may introduce new tolerability challenges, particularly for individuals who already experience gastrointestinal side effects with GLP-1 therapy.
For people doing well on semaglutide, the benefit-risk tradeoff of layering additional peptide-based satiety signaling may not be favorable based on current published PYY1875 results. For people who feel stalled, discussions often benefit from clarifying what “success” means, what side effects are acceptable, and what markers beyond weight are being tracked.
3 Practical Tips
- Pay attention to side effects as much as the scale. Appetite suppression that comes with persistent nausea rarely supports long-term adherence.
- Track patterns, not just weight. Hunger timing, meal satisfaction, and food tolerance changes can provide clearer signals than weekly weigh-ins.
- Discuss expectations openly. Any add-on discussion should include clear goals and stop points if benefit does not outweigh discomfort.
FAQ
What makes PYY different from GLP-1?
PYY and GLP-1 act through different receptors and are being studied as complementary signals in obesity pharmacotherapy. The PYY1875 clinical program focused on a long-acting PYY3–36 analogue and evaluated it alone and as an add-on to semaglutide in randomized studies.
Is PYY1875 available as a prescription medication?
PYY1875 has been studied in clinical trials, including a randomized add-on program with semaglutide, but it is not approved or commercially available as a prescription medication based on the published evidence discussed here.
Does this mean combination therapies will not work?
No. The published PYY1875 results indicate modest efficacy as an add-on to semaglutide and highlight tolerability limits, especially with higher-dose escalation. Those findings inform how future combinations might be designed and tested.
Should patients wait for “the next version” of PYY drugs?
Waiting only makes sense if future studies demonstrate clearer added benefit with acceptable tolerability. Current published data for PYY1875 emphasize modest incremental effects and gastrointestinal tolerability constraints as key issues.
Looking Ahead Without Overpromising
Research into long-acting PYY analogs reflects a broader push to study obesity treatments that combine complementary mechanisms. The current published PYY1875 evidence shows why careful dose design and tolerability are central to whether an add-on strategy can deliver meaningful value for patients.
This article was written by a clinical research–focused medical writing team that reviews peer-reviewed obesity pharmacotherapy literature, including randomized trials and regulatory disclosures, to help patients interpret emerging therapies without hype. Content is developed for educational purposes and grounded in publicly available scientific evidence.
Questions? We are here to help! Call 239-355-3294.