Takeaways
-
Semaglutide significantly improves liver inflammation, fibrosis, and weight loss in patients with MASH.
-
The ESSENCE Phase 3 trial demonstrated superior outcomes for semaglutide over placebo in multiple clinical measures.
-
Integrating semaglutide into a personalized care plan enhances metabolic and liver health outcomes.
Understanding MASH and Its Clinical Challenges
Metabolic dysfunction-associated steatohepatitis (MASH) is a severe liver condition tied closely to obesity and insulin resistance. It involves liver inflammation, fat accumulation, and progressive scarring known as fibrosis. Unlike earlier terms like NAFLD or NASH, MASH reflects clearer diagnostic links to metabolic health. This condition increases the risk of cirrhosis, cardiovascular disease, and liver cancer. As its prevalence grows, so does the urgency for reliable treatment options.
Introducing Semaglutide: Beyond Diabetes Management
Semaglutide was initially developed to treat type 2 diabetes by improving insulin sensitivity and reducing appetite. Over time, researchers discovered that it also helped patients lose significant amounts of weight. These benefits sparked interest in its potential to reduce liver fat and inflammation. Through GLP-1 receptor activation, semaglutide supports multiple metabolic functions. It now stands out as a promising tool in the fight against MASH.
Breakthrough Evidence: What the ESSENCE Trial Revealed
The ESSENCE Phase 3 trial evaluated semaglutide in patients with biopsy-confirmed MASH and liver fibrosis. In this landmark study, 62.9% of patients showed resolution of liver inflammation, while fibrosis improved in 36.8% of cases. These results far outperformed those of the placebo group, marking a potential breakthrough. The study followed over 800 participants across 72 weeks, demonstrating sustained histological and metabolic improvements. Semaglutide may soon shift treatment standards for MASH.
To help visualize semaglutide’s clinical impact, the table below compares outcomes from the ESSENCE Phase 3 trial. These data points highlight how semaglutide outperformed placebo in treating liver-related and metabolic markers in MASH patients.
| Outcome Measure | Semaglutide Group | Placebo Group |
|---|---|---|
| Steatohepatitis Resolution (without fibrosis worsening) | 62.9% | 34.3% |
| Liver Fibrosis Improvement (without steatohepatitis worsening) | 36.8% | 22.4% |
| Combined Resolution & Fibrosis Improvement | 32.7% | 16.1% |
| Average Weight Loss | 10.5% | 2.0% |
How Semaglutide Impacts the Liver at a Biological Level
Semaglutide’s impact goes deeper than weight loss; it influences liver biology at a cellular level. By reducing de novo lipogenesis, it lowers liver fat accumulation significantly. It also decreases liver inflammation by modulating immune cell activity and oxidative stress. These mechanisms collectively reduce ballooning degeneration, a hallmark of MASH pathology. Fibrosis regression appears tied to suppressed fibrogenic signaling and reduced stellate cell activation. Supporting your liver with antioxidant-rich IV therapy may complement the metabolic improvements initiated by semaglutide.
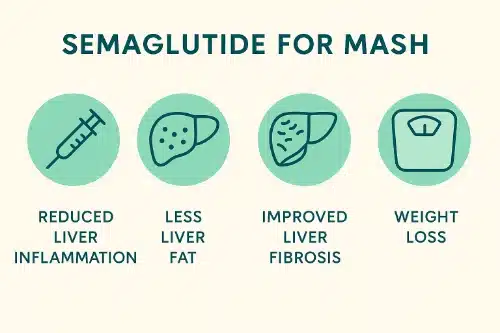
Infographic: Key Benefits of Semaglutide in MASH Treatment
Weight Loss, Metabolic Gains, and Liver Health
Weight loss from semaglutide therapy correlates directly with improved liver outcomes. As patients shed visceral fat through physician-guided weight loss, insulin sensitivity improves and liver enzymes normalize. This chain reaction reduces hepatic fat and inflammatory markers in the bloodstream.
Better glycemic control and reduced triglyceride levels reinforce these liver benefits. At Fountain of Youth SWFL, we monitor these indicators closely to personalize treatment plans for patients with metabolic liver conditions.
Comparing Semaglutide to Other MASH Therapeutics in Development
Several drugs are in development for MASH, yet semaglutide holds a unique position. Many alternatives focus solely on liver fibrosis or metabolic parameters. Semaglutide addresses both, offering dual-action benefits that others may lack. Its efficacy in reducing both inflammation and fibrosis exceeds that of most competitors in clinical trials. For patients seeking comprehensive improvement, semaglutide offers a more holistic approach.
Safety and Tolerability in MASH Patients
Semaglutide has a well-characterized safety profile, even outside diabetic populations. Gastrointestinal effects such as nausea and constipation appear most frequently but usually diminish over time. In the ESSENCE trial, serious adverse events occurred at the same rate in both semaglutide and placebo groups. This reinforces the drug’s tolerability among broader patient populations. At Fountain of Youth SWFL, we counsel patients on side effect management and tailor dosing schedules as part of our doctor-supervised medical aesthetics care.
Regulatory Status and Future Availability
As of 2025, semaglutide is not yet FDA-approved specifically for MASH, though its approval may be imminent. The strong evidence from phase 3 trials supports a new indication filing with regulatory agencies. Experts expect final decisions as more long-term data becomes available through extended study periods. Wider availability could reshape treatment guidelines and increase access for high-risk populations. Patients should stay informed about emerging updates.
Who Might Benefit Most from Semaglutide Therapy?
Individuals with moderate fibrosis and signs of metabolic syndrome are ideal candidates for semaglutide. Those who struggle with weight management and insulin resistance may also respond well. Older adults with co-existing conditions may require additional monitoring but can still benefit significantly. Semaglutide may not suit patients with severe gastrointestinal issues or prior adverse reactions. Patients already exploring hormone balance therapies may find added synergy with semaglutide for metabolic improvement.
Integrating Semaglutide Into MASH Management Plans
Effective MASH treatment goes beyond prescribing a single drug and often includes personalized wellness treatments for long-term success. Semaglutide should be part of a broader, integrated care plan that includes nutrition, physical activity, and regular monitoring. Healthcare teams must track weight, liver enzymes, and imaging results throughout the treatment process. At Fountain of Youth SWFL, we offer coordinated care between our metabolic and liver specialists to ensure comprehensive management. This strategy enhances both compliance and long-term results.
Frequently Asked Questions
Is semaglutide currently approved for treating MASH?
No, it’s not FDA-approved for MASH yet, but late-stage trials suggest approval is likely soon.
Can patients without diabetes take semaglutide for liver disease?
Yes, non-diabetic patients in trials showed strong responses, making it viable for broader use.
How soon do improvements in liver health appear?
Many patients see benefits by week 24, with continued gains into the second year of treatment.
Will semaglutide replace liver biopsy as a monitoring tool?
Not entirely, but improvements in non-invasive biomarkers may reduce biopsy reliance over time.
3 Practical Tips for Patients Considering Semaglutide for MASH
- We recommend patients track weight, liver enzymes, and metabolic markers monthly during therapy.
- Reducing alcohol, refined carbs, and saturated fats will help semaglutide work more effectively.
- Lastly, patients should consult with a provider who understands both liver pathology and metabolic health for optimal results.
Looking Ahead: What This Could Mean for Liver Medicine
Semaglutide’s success in MASH represents a shift toward treating liver disease through systemic metabolic improvements. This approach supports both liver regeneration and overall health transformation. As more patients access semaglutide, outcomes may improve across multiple chronic conditions. The therapy’s dual benefits reduce the need for separate medications. With expert care and early intervention, we can help patients reclaim liver health and prevent future complications.
VCU-led research further reinforces semaglutide’s ability to address liver damage and underlying metabolic dysfunctions. Yale School of Medicine highlights the biological mechanisms driving liver fat reduction in GLP-1 therapies, supporting these transformative outcomes.
Medical review: Reviewed by Dr. Keith Lafferty MD, Fort Myers on May 16, 2025. Fact-checked against government and academic sources; see in-text citations. This page follows our Medical Review & Sourcing Policy and undergoes updates at least every six months.