Takeaways
-
RPN-001 is an oral, fertility-preserving alternative to injectable testosterone that stimulates natural hormone production without suppressing sperm count.
-
Clinical trials show promising improvements in testosterone levels and motile sperm counts, with a favorable safety profile over 16 weeks.
-
The therapy could redefine male infertility treatment by offering a non-hormonal path to restore testosterone and support conception.
A Non-Hormonal Approach to Boosting Testosterone and Sperm Health
Understanding the Unmet Need in Male Reproductive Health
Male infertility rates continue climbing in the United States and abroad. Toxic exposures, metabolic disorders, and ineffective medical weight loss programs disrupt endocrine balance and impair sperm quality. The AUA-ASRM guideline urges thorough hormone evaluation before selecting therapy. Despite this advice, many clinicians still begin treatment with injectable testosterone. That approach lifts energy yet almost eliminates internal sperm production during active dosing. Couples then pause therapy, endure symptoms again, and wait months for recovery.
What Is RPN-001?
RPN-001, or leflutrozole, represents a next-generation oral aromatase inhibitor engineered specifically for male infertility. Developers calibrated micro-dosing so estrogen declines just enough to trigger natural testosterone rebound without bone risks. Early Phase 2a work showed weekly capsules normalized free testosterone within eight weeks in obese hypogonadal men. Participants also reported improved mood, energy, and libido, further supporting the approach. Unlike oncology AIs, RPN-001 targets balance rather than complete estrogen suppression. Clinicians therefore view the drug as a fertility-first therapy, not a performance enhancer.
How RPN-001 Works Inside the Male Body
How RPN-001 Works Inside the Male Body
The Aromatase Inhibition Mechanism
Aromatase converts circulating testosterone into estradiol, completing a feedback loop that slows pituitary activity. Moderate enzyme blockade with RPN-001 lifts luteinizing hormone and follicle-stimulating hormone secretion.
These gonadotropins travel to the testes and ignite endogenous testosterone synthesis. Hormone rhythms stay closer to physiologic patterns than seen with injections. For deeper mechanism detail, researchers outline the cascade in a comprehensive review article.
Impact on Spermatogenesis
Elevated gonadotropins activate Sertoli and Leydig cells, which support germ-cell maturation and androgen output. Investigators observed significant gains in total motile sperm counts after sixteen weeks on study drug. Semen volume and progressive motility improved alongside stable intratesticular testosterone levels. These findings contrast sharply with data from patients using injectable hormones. Preserving that microenvironment keeps fertility goals realistic during androgen optimization.
Inside the Phase 2 U.S. Clinical Trial
Trial Design and Key Endpoints
The current Phase 2 study randomizes about two hundred men into three dosing arms and a placebo group. Participants swallow weekly capsules for sixteen weeks under double-blind conditions to minimize bias. Total motile sperm count at week sixteen serves as the primary endpoint. Secondary measures include free testosterone, DNA fragmentation, libido scores, and quality-of-life assessments. Monitoring also captures safety markers like hematocrit, liver enzymes, and mood changes throughout follow-up.
Patient Selection and Inclusion Criteria
Eligible volunteers are eighteen to forty-nine years old with low free testosterone and abnormal semen parameters. Screening removes candidates with varicoceles, endocrine disorders, or recent anabolic-steroid exposure to avoid confounding. Baseline DEXA scans provide a reference for bone density shifts during therapy. Participants discontinue external testosterone eight weeks before randomization to reset hormonal baselines. Ongoing counseling supports adherence and clarifies expectations for fertility timelines.
Earlier Findings: What Preclinical and Phase 2a Data Showed
Animal studies demonstrated dose-dependent testosterone rises without testicular shrinkage or organ toxicity. A randomized crossover trial published in the European Journal of Endocrinology found hormone normalization within eight weeks. Motile sperm counts increased nearly thirty percent, while energy and libido scores also improved. Headaches and temporary hematocrit elevations represented the most common adverse events and resolved spontaneously. Researchers considered the safety profile acceptable for expansion into larger randomized studies now underway.
How RPN-001 Compares to Injectable Testosterone Therapies
Injectable testosterone floods circulation with exogenous androgen and suppresses pituitary feedback almost immediately. Sperm production stalls, forcing couples to suspend therapy when pursuing pregnancy. Many men have tried pellet therapy seeking steadier hormone delivery, yet fertility outcomes remain similar. RPN-001 instead boosts endogenous testosterone while allowing spermatogenesis to continue uninterrupted. Once-weekly oral dosing eliminates needles, disposal hassles, and frequent clinic visits.
This table outlines key differences between RPN-001 and other treatment options for men facing both testosterone deficiency and fertility concerns.
Comparison of Common Male Fertility Therapies
| Therapy | Delivery Method | Supports Fertility | Requires Monitoring |
|---|---|---|---|
| RPN-001 (leflutrozole) | Oral capsule, once weekly | Yes – preserves sperm production | Yes – hormones, bone density, bloodwork |
| Injectable Testosterone | Intramuscular injection, weekly or biweekly | No – suppresses sperm production | Yes – hematocrit, PSA, liver enzymes |
| Clomiphene Citrate | Oral tablet, daily or every other day | Yes – stimulates endogenous testosterone | Yes – estradiol, free-T, semen analysis |
| hCG Monotherapy | Subcutaneous injection, several times per week | Yes – mimics LH to stimulate testes | Yes – T-levels, testicular size, estradiol |
Safety, Monitoring, and Long-Term Considerations
Short-Term Tolerability
Headaches, mild flushing, and transient hematocrit increases appeared in early cohorts but resolved quickly. Regular blood panels help clinicians detect laboratory shifts before symptoms escalate. Our practice integrates on-site phlebotomy, streamlined results, and supportive vitamin injections to ease treatment. Rapid feedback empowers patients to adjust hydration, nutrition, or dosage under professional guidance. Such proactive care keeps progress steady while minimizing daily inconvenience.
Bone Health and Estrogen Suppression Risks
Estrogen protects male skeletal tissue by regulating bone turnover and calcium absorption. A detailed Endotext review outlines fractures observed when estrogen falls too low for extended periods. RPN-001 dosing maintains mid-normal estradiol, reducing that long-term risk in most participants. Baseline and annual DEXA scans confirm any skeletal trends and guide supplement recommendations. Weight-bearing exercise and adequate vitamin D intake further strengthen bones during therapy.
A Look at Market Disruption and Regulatory Momentum
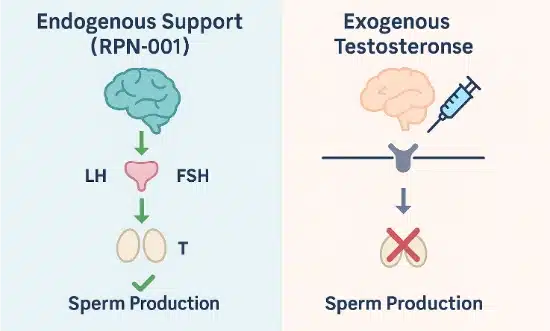
This visual compares how RPN-001 supports hormone-driven sperm production, while exogenous testosterone disrupts that natural feedback loop.
No FDA-approved oral therapy currently addresses male infertility caused by secondary hypogonadism. RPN-001 therefore enjoys first-mover status in a market dominated by injections. Analysts project strong adoption among urologists, fertility specialists, and integrative men’s health clinics. Breakthrough-therapy designation could accelerate review if pivotal data confirm significant benefit. Competitive pressure may spur refinement of adjacent fertility-preserving hormone strategies.
Where RPN-001 Fits in the Male Fertility Toolkit
Clinicians can pair RPN-001 with low-dose hCG to amplify intratesticular testosterone when counts plateau. Lifestyle pillars such as sleep quality, micronutrient sufficiency, and progressive resistance training remain essential.
Fountain of Youth SWFL integrates nutrition counselling, rejuvenation and healing therapies, and pharmacology for holistic care. Regular semen analyses every three months guide personalized dose adjustments rather than fixed schedules. Such flexibility reflects modern, patient-centric endocrine practice and supports long-term family planning.
FAQ: Common Questions About RPN-001
Is RPN-001 designed to replace testosterone therapy altogether?
Many men with fertility goals may use RPN-001 instead of injections and maintain symptom relief. Clinicians reassess hormone status after conception to decide ongoing management.
Can RPN-001 be used by men with normal testosterone but poor sperm quality?
Doctors first explore anatomical issues, infections, or lifestyle barriers before choosing endocrine solutions. If subtle hormonal imbalance contributes, low-dose RPN-001 may provide additional benefit.
What makes RPN-001 safer than anabolic steroids or performance-enhancing drugs?
The drug fine-tunes physiologic pathways rather than delivering synthetic hormones at supraphysiologic levels. Cardiovascular, mood, and testicular risks therefore remain significantly lower.
How long would someone typically stay on RPN-001 treatment?
Clinical trials last sixteen weeks, yet real-world courses often continue until pregnancy occurs. Ongoing labs inform responsible discontinuation timing and minimize relapse risk.
3 Practical Tips for Men Exploring Fertility-First Testosterone Support
- Obtain a complete hormone panel, including estradiol and prolactin, before starting any endocrine therapy.
- Commit to three weekly sessions of resistance training, which synergizes with hormonal treatments to elevate testosterone naturally.
- Ask your provider about enrolling in clinical trials to access cutting-edge therapies under expert supervision.
Why This Matters Going Forward
RPN-001 signals a shift toward fertility-friendly hormone optimization that keeps reproductive ambitions intact. Upcoming Phase 2 readouts will clarify optimal dosing, safety margins, and population responsiveness. Positive findings could reshape clinical guidelines and give hope to couples navigating male-factor infertility. Stakeholders across urology, endocrinology, and reproductive medicine watch these milestones with heightened anticipation. Our team stands ready to translate emerging evidence into personalized care pathways as soon as regulators approve.
Medical review: Reviewed by Dr. Keith Lafferty MD, Medical Director at Fountain of Youth SWFL on July 19, 2025. Fact-checked against government and academic sources; see in-text citations. This page follows our Medical Review & Sourcing Policy and undergoes updates at least every six months. Last updated September 15, 2025.